
Katie Babcock

“Researchers knew that mutations in a specific gene caused 40 per cent of inherited cases of ALS, but there are few studies of the normal function of this gene,” said Janice Robertson, professor in the Faculty of Medicine’s Department of Laboratory Medicine and Pathobiology. “Other scientists have focused on how the gene’s mutation causes disease. We’ve developed the first antibodies to track what this gene does in both a normal and diseased cell.”
Using these antibodies, Robertson and her team tracked proteins from the key gene, C9orf72. They discovered that a specific protein from this gene might help transport other essential proteins in and out of a motor neuron cell’s nucleus, the command centre of the cell. Their findings were recently published in the Annals of Neurology.
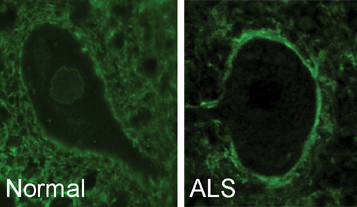 “We saw that a protein from C9orf72 normally surrounds the nucleus of a motor neuron cell,” said Robertson, who is also a scientist at U of T’s Tanz Centre for Research in Neurodegenerative Diseases. “But in ALS or FTD, this protein moves to the outer membrane of the cell. When this protein is misplaced, it can’t help other proteins move in and out of the cell’s nucleus and the cell dies.”
“We saw that a protein from C9orf72 normally surrounds the nucleus of a motor neuron cell,” said Robertson, who is also a scientist at U of T’s Tanz Centre for Research in Neurodegenerative Diseases. “But in ALS or FTD, this protein moves to the outer membrane of the cell. When this protein is misplaced, it can’t help other proteins move in and out of the cell’s nucleus and the cell dies.”
This transportation normally depends on a cascade of events that acts like a relay race. DNA, the cell’s instructions, is converted into RNA which then makes proteins for the cell that allow it to carry out its functions. Just like a relay, when a protein from C9orf72 disappears from surrounding the nucleus, it can no longer help transport other essential proteins that keep the cell alive.
Next, the researchers want to understand how C9orf72 is involved in transporting these proteins and potentially target the pathway with drugs.
“This pathway is easily targeted with drugs that already exist,” said Dr. Shangxi Xiao, first author of the publication. “If we can restore function to this pathway and make proteins go back to the nucleus, we could develop treatment options.”
Robertson’s remarkable findings provide hope for treating ALS and FTD. ALS is a neuromuscular disease that begins as a mild muscle weakness and progresses to complete paralysis. Frontotemporal dementia is closely linked to ALS and leads to memory loss, behavior changes and difficulties with movement and speech.
“James Hunter is one of our financial supporters, and he’s completely paralyzed,” said Robertson. “He uses his eyes to communicate through a computer program, but now his eyes have almost stopped moving — this is ALS.”
Robertson believes the impact of this and others’ research will revolutionize treatment for ALS and FTD. “Recently, there have been four important publications, all honing in on this specific pathway. We’re getting closer to finding treatment options,” she said. “We’d like to thank those involved in the James Hunter and Family ALS Initiative and the families who have donated tissues for research. Ultimately, our goal is to find a treatment for patients who desperately need more options.”

